Learning Objectives
By the end of this topic, the student should be able to:
- Describe the endogenous opioid system and its physiological roles.
- Describe the mechanism of action of opioid agonists.
- Explain how opioids can act in the central nervous system and influence opioid use disorder.
- Recognize the basic neuroscience of opioid use.
Key Concepts
- The brain produces several endogenous peptide opioid agonists that activate three receptors: µ (mu), δ (delta), and κ kappa.
- The opioid system exerts its activity across multiple physiological systems, including the regulation of pain.
- In the central nervous system, opioid receptors are involved in reward system signalling, which can influence the development of opioid use disorder.
- Opioid use disorder is treatable, both with opioid agonist treatment (OAT), as well as therapy-based and other treatments.
- Opioid receptors are found in many brain regions, but in the context of understanding opioid-use disorder, the greatest focus is on the ventral tegmental area (VTA), the nucleus accumbens (NAc), and the locus ceruleus (LC).
Endogenous Opioids
When most people think of opioids, they usually think of medicines or unregulated drugs. In fact, the human body produces opioids as part of its natural neuroregulation processes. Opioids produced in the body are considered endogenous opioids. This is in contrast to exogenous opioids, which are opioids one might use for medicinal or other purposes. Exogenous opioids are either plant-derived or synthetic.
Test Your Knowledge
How many opioids are produced in the human brain (endogenous opioids)?
Types of Endogenous Opioids
Plant-derived or synthetic opioids are all large organic molecules; however, endogenous opioids are small peptides (i.e., made up of amino acids). These opioid peptides are derived from larger proteins (preproopiomelanocortin, preproenkephalin, and preprodynorphin).
There are three main types of endogenous opioid peptides:
- endorphins
- enkephalins
- dynorphins
Opioid Receptors in The Body
Both endogenous opioid peptides and exogenous opioids (medicines, drugs) act primarily via three opioid receptors:
- µ (mu),
- δ (delta), and
- κ (kappa).
Although all three opioid receptors are expressed throughout the body and across many systems, mu opioid receptors are sometimes referred to as supra-spinal because they mediate many of the effects in the brain, including acting in the reward system (i.e., limbic system).
Most opioids are considered full agonists, which means they can maximally activate opioid receptors. Some are partial agonists that only partially activate receptors.
Full agonists examples:
- morphine,
- oxycodone,
- fentanyl, and many others.
Partial agonist example:
- buprenorphine
Mechanism of Action—Pain
Opioids are used primarily for their pain-relieving (analgesic) effects for moderate to severe acute pain due to a variety of causes.
Pain is signaled to the brain via activation of the ascending pain pathway.
- The ascending pain pathway delivers the pain signal from the source of the pain to the brain.
- Activation of opioid receptors inhibits this pathway.
The body (including the opioid system) is able to dampen pain by activating the descending inhibitory pathway. This pathway works from the brain down to the spinal cord, so works in the opposite direction of the ascending pain pathway. The pathway inhibits the ascending pain signal.
The Brain’s Reward System
The limbic system is a collection of areas in the brain that control or influence things like emotions, motivation, and memory.

Richfield, D. (2015). Mesolimbic Pathway. Wikimedia. https://commons.wikimedia.org/wiki/File:Mesolimbic_pathway.svg and licensed under (CC BY-SA 4.0).
Within the limbic system, the nucleus accumbens is a structure that is sometimes called the reward centre because it influences reward, reinforcement, and aversion. For example, different types of rewarding activities—drugs, sex, gambling, video games—all result in reward system activation. A common physiological response to these is the release of the neurotransmitter dopamine.

Substances including nicotine, opioids, and others stimulate the reward centre. At the same time, the limbic system is consolidating memories. This makes you remember where you were, who you were with, and what you did when your reward system was activated. Activating the reward centre and forming these memories can influence your motivation to pursue similar rewards in the future.
NOTE: The biology happening within your limbic system is a key factor, but not the only factor, in determining whether a rewarding activity such as substance use will be a one-time experiment, an infrequent activity, or a regular activity, or might lead to addiction/substance use disorder.
To learn more about the genetics and epigenetics of opioid use and use disorder, see Module 1, Topic D.
Treatment of Opioid Use Disorder
Opioid agonist therapy or treatment (OAT) is the primary treatment for opioid use disorder or problematic opioid use.
- The goals of therapy include replacing problematic opioid use with regular use of specific opioids and doses, prescribed by a physician, and avoiding symptoms of withdrawal.
Compounds Used for OAT
Methadone
Methadone is a full agonist at the opioid receptor, and a longer-acting opioid.
Buprenorphine
Buprenorphine is a partial agonist at the opioid receptor meaning that it activates the receptor, but not maximally. Thus, in the presence of a full agonist, it actually acts as an opioid receptor blocker, and can even induce opioid withdrawal symptoms.
Effectiveness of OAT

- Methadone does better by some measures in clinical trials (73 percent retention in treatment versus 22 percent given placebo) compared to buprenorphine/naloxone (64 percent retention in treatment versus 39 percent given placebo) (Amato et al., 2013).
- In Canada, a combined formulation of buprenorphine and naloxone is the first-line OAT (Bruneau et al., 2018) because of its established efficacy and safety, and because it does not require additional prescriber training like methadone.
Other Treatments of Opioid Use Disorder
In addition to OAT, emerging pharmacological therapies, therapy-based treatments, support groups, rapid access addiction clinics, residential treatment, and other treatments also demonstrate various degrees of efficacy (Bruneau et al., 2018; Leshner & Mancher, 2019; Taha, 2018).
- Slow-release oral morphine formulations are used (in Canada, typically used after trying buprehorphine/naloxone and methadone).
- Naltrexone (typically extended-release) is an opioid receptor antagonist like naloxone that reduces cravings for opioids (and other drugs including alcohol) and is typically used in recovery.
- Cognitive behavioural therapy, behavioural therapy, contingency management approaches, and motivational interviewing are typically used in conjunction with OAT.
Please take the time to explore the following successful recovery story.
The Neuroscience of Opioid Use
Let us review some of the ways opioid drugs affect the brain, as well as the basis for opioid tolerance and withdrawal.
Opioids travel through the bloodstream to the brain. Once in the brain, they bind to opioid receptors on the surfaces of opiate-sensitive neurons.
Opioid receptors are found in many brain regions, but in the context of understanding opioid-use disorder, the greatest focus is on the following three regions:
- ventral tegmental area (VTA),
- nucleus accumbens (NAc), and
- the locus ceruleus (LC).
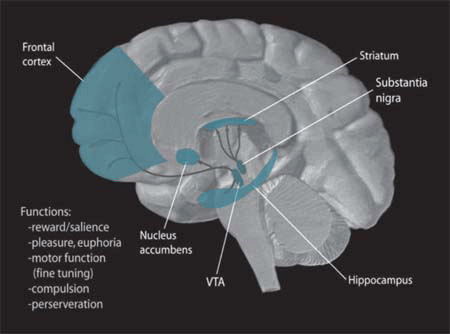
In the brain, dopamine plays an important role in the regulation of reward and movement. As part of the reward pathway, dopamine is manufactured in nerve cell bodies located within the ventral tegmental area (VTA) and is released in the nucleus accumbens and the prefrontal cortex. Its motor functions are linked to a separate pathway, with cell bodies in the substantia nigra that manufacture and release dopamine into the striatum.
NIDA. (2006). Dopamine Pathways [Image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Dopamine_Pathways.png

Diego69. (2010). Locus Coeruleus [Image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Locus-coeruleus.gif and licensed under (CC BY-SA 3.0
Let us take a look at the processes and structures involved in opioid use one at a time.
- The VTA and the NAc are part of the mesolimbic (midbrain) reward system. Opioid signalling activates the VTA, which releases the chemical dopamine (DA) in the NAc. This causes feelings of euphoria. The induced pleasure is what typically motivates the sustained use of opioids.
- Over time, the opioid receptors can become desensitized, which means an increased opioid dose is needed to stimulate the VTA brain cells to release the same amount of DA in the NAc. This means a bigger opioid dose is needed to produce a comparable amount of pleasure.
- Neurons in the LC produce noradrenaline (NA, also called norepinephrine, NE), which regulates wakefulness, breathing, blood pressure, and metabolism, among other functions. In the LC, opioids suppress the release of NA, resulting in symptoms such as drowsiness, slowed respiration, and low blood pressure. However, with repeated opioid exposure, LC neurons compensate for the opioid induced suppression by increasing NA release. As a result, when opioids are not present to suppress the increased activity of LC neurons (increased NA release), the resulting excessive NA levels trigger jitters, anxiety, muscle cramps, and diarrhea.
- The repeated exposure to escalating opioid doses lead to gradual but long-lasting effects on the brain. For example, to counter the increase in dopamine that opioid-use induces, the brain starts to naturally produce less dopamine through the mesolimbic reward system. This means that the drug user experiences less pleasure with everyday activities that would have previously produced pleasure.
These neurochemical changes can drive addiction as an individual becomes dependent on opioids to maintain normalcy and standard function.
Pharmacological interventions involve the introduction of molecules that bind the opioid receptors e.g., methadone and buprenorphine, but elicit different effects.
The Importance of Recovery Stories

dusanpetkovic/iStock
Recovery stories are important to hear and share as they have multiple purposes. Stories of recovery can challenge the dominant or mainstream construction of who is an addict. Recovery stories:
- provide hope for others,
- represent a verbal or written testimony to the strength and resiliency of the recovery process, and
- by sharing, involve others who then witness the recovery of the individual.
Individuals who share recovery stories can speak to the feeling of control and mastery over a phenomenon that had earlier dominated their destiny or life.
Recovery stories allow the individual to author their own narrative, which can be a very empowering process, and provides the individual the opportunity to contextualize the intrapersonal, interpersonal, societal, cultural, and political contributors to a substance use disorder.
This is especially important for Indigenous Peoples and members of marginalized groups. The effects of colonization, racism, oppression, and other distal factors are known to contribute to a substance use disorder.
Below are two stories shared by individuals who challenge the stereotypical view of who becomes addicted and of what their recovery process looks like.
Lived recovery experiences
The first story is by Tara Conner, former Miss USA 2006, who now is a recovery advocate and speaks to audiences throughout the United States.
This second recovery story is told by a physician who has chosen to remain anonymous. His recovery story highlights the multiple and intersecting domains of work, home, family, professional practice, friends, and community.
Please take a moment to read the article A Personal Story of Addiction.
Stop and Think
Now that you have reviewed this content, consider the following:
Choose one of the recovery stories linked to in this module. Reflect on how these stories could impact a person experiencing opioid use disorder.
Questions
References
Amato, L., Minozzi, S., Davoli, M., & Vecchi, S. (2013). Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database of Systematic Reviews, 10, CD004147.
Bruneau, J., Ahamad, K., Goyer, M.-E., Poulin, G., Selby, P., Fischer, B., Wild, C., & Wood, E. (2018). Management of opioid use disorders: A national clinical practice guideline. Canadian Medical Association Journal, 190, E247–E257.
Brunton, L. L., Hilal-Dandan, R., & Knollmann, B. C. Goodman and Gilman’s the pharmacological basis of therapeutics (13 ed.). McGraw-Hill Education.
Hammer, R. R., Dingel, M. J., Ostergren, J. E., Nowakowski, K. E., & Koenig, B. A. (2012). The experience of addiction as told by the addicted: Incorporating biological understandings into self-story. Culture, Medicine and Psychiatry, 36(4), 712–734. https://doi.org/10.1007/s11013-012-9283-x
Katzung, B. G. (2018). Basic and clinical pharmacology (14 ed.). McGraw-Hill Education.
Kosten, T. R., & George, T. P. (2002). The neurobiology of opioid dependence: Implications for treatment. Science & Practice Perspectives, 1(1), 13–20. https://doi.org/10.1151/spp021113
Leshner, A. I., & Mander, M. (Eds.). (2019). Medications for opioid use disorder save lives. A consensus study report. National Academies of Sciences, Engineering, Medicine.
Taha, S. (2018). Best practices across the continuum of care for the treatment of opioid use disorder. Canadian Centre on Substance Use and Addiction.
Wang, S.-C., Chen, Y.-C., Lee, C.-H., Cheng, C.-M. (2019). Opioid addiction, genetic susceptibility, and medical treatments: A review. International Journal of Molecular Sciences, 20, 4294.
Wiklund, L. (2008). Existential aspects of living with addiction–Part 1: Meeting challenges. Journal of Clinical Nursing, 17(8), 2426–2434.https://doi.org/10.1111/j.1365-2702.2008.02356.x